Clinical immersion programs are elective courses that offer continuing education for various life science teams as well as practicing clinicians. These programs are traditionally held in a clinical setting and meant to train attendees on a range of topics, including disease state education, competitive landscape, treatment patterns, and expert perspectives in the management of cancer patients. All these things inform diagnosis and treatment decision-making and ultimately support better-quality care. This is the second post in a series on clinical immersion programs. Find the first article here.
Course content is fully customizable on the basis of attendee needs, and clinical immersion programs are consistently ranked as top educational training tools for the life science industry. They are often developed in partnership with centers of excellence or community practices. Most healthcare organizations and life science companies rely on these programs to keep their employees up-to-date and to bridge gaps in knowledge, as well as to fulfill continuing education requirements.
Clinical Immersion Programs for Pharma
Clinical immersion programs are a necessity for pharmaceutical companies. While some employees have a medical background, others have limited experience in a clinical setting. Immersion courses in pharma are usually geared toward drug discovery and development, including both the scientific and regulatory aspects of these activities.
Players in the pharmaceutical space need to understand the clinical context of their products: they should be able to share knowledge about a drug’s delivery mechanism, mode of action, or potential adverse effects. Sales teams, for example, often need to articulate their understanding of a product in conversations with physicians. The ability to communicate nuances helps them stand out.
Aptitude Health, which specializes in oncology and hematology, has delivered clinical immersion programs to internal pharma staff, including sales teams and medical science liaisons. While it is critical for a pharma company to develop a savvy sales team, other needs for immersion training in the pharma environment include onboarding faculty with new network capabilities and informing them of new regulatory practices.
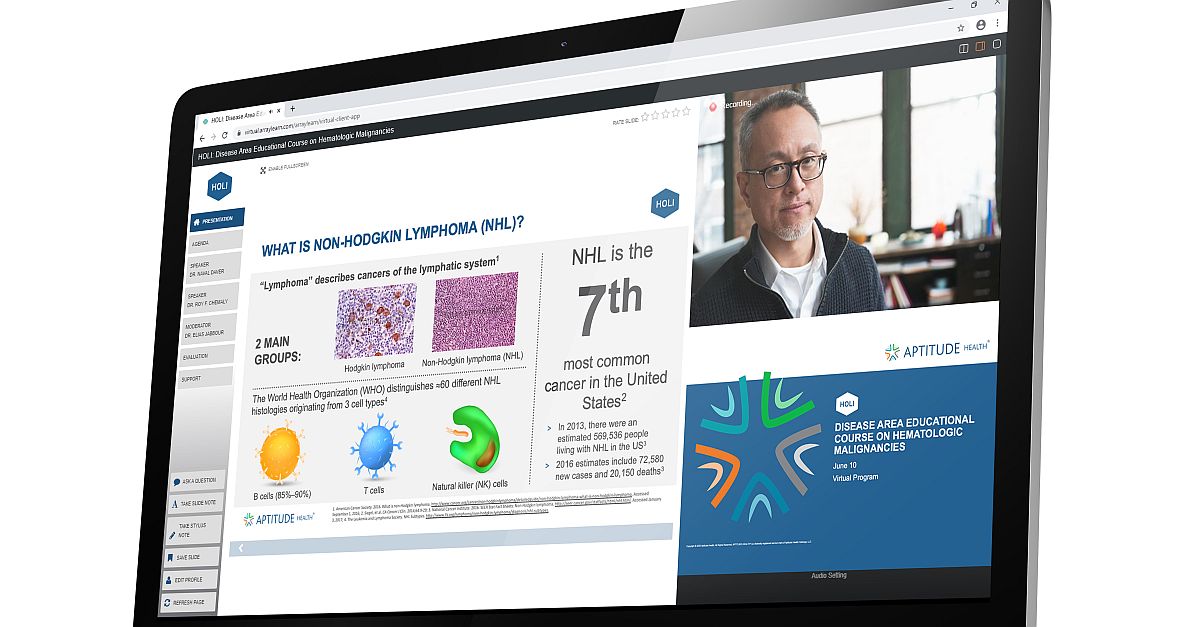
Digital Versions Are the New “Normal”
During the COVID-19 pandemic, in-person sessions were abandoned in favor of digital solutions. Virtual training platforms indeed proved to be a cost-effective alternative and supported increased accessibility for attendees, who could join a session from anywhere. In fact, this new “normal” has demonstrated additional benefits.
Virtual capabilities allow more flexibility in scheduling training sessions, without hurdles like reserving a space, organizing/buying additional resources for a classroom, and accommodating a host of logistic needs. Utilizing digital platforms, a company can plan and implement a clinical immersion program on the fly, say to prepare staff for an upcoming product launch or to share brand-new data following a conference or meeting.
Digital courses are formatted according to the goals of the client and may include interactive presentations, case study workshops, competitive simulations, role-play exercises, and panel discussions. To get a more realistic picture of how clinical immersion programs can be utilized for a pharma audience, let’s look at some examples from Aptitude Health’s Hematology Oncology Learning Institute (HOLI).
Case Study Example 1: Clinical Immersion Program
Let’s consider an example scenario of a successful clinical immersion program. Aptitude Health was asked to design a digital training course for a pharma client who had acquired a poly(ADP-ribose) polymerase (PARP) inhibitor from another company. The goal of the HOLI program was to educate the client’s global medical teams on monotherapy and combination therapy of PARP inhibitors in prostate cancer.
The HOLI clinical immersion program was structured as 2 half-day sessions that ran for 3 hours each day. The first day focused on monotherapy indications of PARP inhibitors; the second day centered on combination therapy. The program featured presentations by 3 globally renowned experts, along with interactive patient case workshop rotations and attendee question-and-answer sessions.
Case Study Example 2: Integrated Delivery Networks
In a second example, we’ll examine a HOLI virtual training program on integrated delivery networks (IDNs). The client commissioned this course to train their new payor-focused team on IDNs. A small but vital team of around a dozen market access employees participated in 2 half-day sessions, including didactic lectures and panel discussions on health and hospital systems, specialty pharmacy, and payor-related topics. Program faculty included PharmDs and administrators from major IDNs.
For this program, Aptitude Health partnered with Excelera to prepare the course content, answering questions like, What are the benefits and challenges of a large healthcare system compared with independent hospitals or private practices? What are the various physician provider models in a hospital system? And what is the role of IDNs in cancer care delivery? In fact, the discussion around IDNs as they influence the practice of oncology was a particular focus of this HOLI program.
Partner With Agencies That Have Program Experience
As training programs increasingly migrate to virtual platforms, they offer an easier way to schedule educational seminars and improve program access to teams. This makes partnering with an experienced agency even more valuable. In today’s landscape, life science companies need to seek longer-term partnerships with agencies that have plenty of experience in organizing digital clinical immersion programs.
Not only should agencies demonstrate expertise in designing and executing clinical programs for virtual platforms, but they should also show a deep commitment to understanding their client’s business. In the pharma space, this means demonstrating familiarity with nuanced processes like pharmacovigilance, adverse events monitoring, and compliance.
An agency with project management prowess is not enough. Subject matter expertise is crucial and can make or break a good pharma-agency relationship. An agency should further deliver access to a host of external experts like global key opinion leaders and health system networks. Backed by unparalleled access to industry experts and market-driving physicians, Aptitude Health is a valuable strategic partner for companies in the oncology market.