During the first 4 months of 2021, Aptitude Health conducted a number of business intelligence CORE meetings with Axess Network community oncologists to gain insights into current practice patterns for managing patients with acute myeloid leukemia (AML). These events are designed to increase understanding of patterns of care, disease-state treatment choices, practice challenges, and resource needs. The questions posed fell into 3 main silos: practice overview, frontline treatments, and management of relapsed/refractory (R/R) disease.
AML practice overview
Community oncologists see a range of patients with AML and use risk stratification to guide treatment.
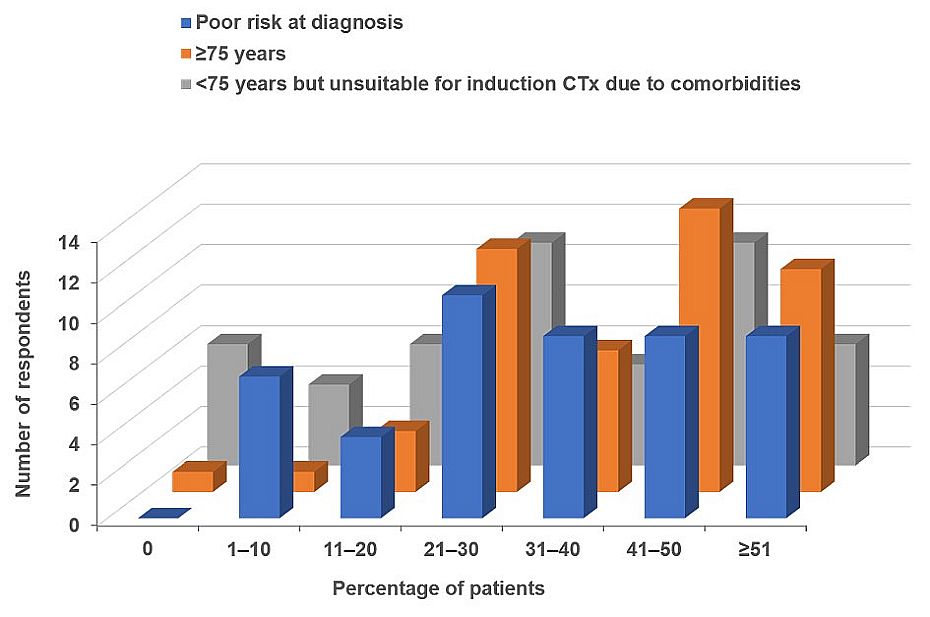
In total, 49 community oncologists participated. The physicians manage between 1 and ≥21 new patients with AML per year, with the majority (57%) seeing between 1 and 5 new patients and 35% currently following between 1 and 5 patients. As would be anticipated, risk stratification for newly diagnosed patients with AML is almost universally performed, with only 3 participants stating that they did not perform this for each patient they saw. All physicians stratify risk on the basis of clinical factors, such as comorbidities; the majority (94%) also used age and genomic and mutational analyses to stratify their patients, while 75% factor in the presence of prior hematologic malignancies. A snapshot of the range of patients whom community oncologists treat in their daily practice is shown in Figure 1.
Figure 1. Characteristics of the patients with AML seen by community oncologists and hematologists
CTx, chemotherapy.
Frontline AML management
Mutational profiling is critical to determining the optimal treatment strategies for patients with AML.
Overall, a regimen featuring venetoclax is the most popular recommendation for the frontline treatment of patients with AML.
The increased understanding surrounding the molecular pathogenesis of AML has been accompanied by a rise in molecular testing to guide physicians to the most appropriate management strategy for the disease. Our data demonstrate there was a high uptake of mutational testing among community oncologists for patients with newly diagnosed AML. Testing for NPM1, FLT3 (-ITD or -TKD), CEBPA, TP53, IDH1, and IDH2 mutations is routinely undertaken, with 86% of oncologists stating that they test for all of these markers. The molecular/genomic analysis is generally outsourced, although 25% reported that the samples are tested in their local hospital, presumably reflecting the capacity and available technology. Most oncologists (76%) receive the results within 8–14 days of testing, although the turnaround time ranged from <7 to 21 days overall. Given the range of time it takes to receive the test results, it is not surprising that most oncologists (73%) consider starting frontline therapy before the results are available, with only 5 participants (10%) stating they never start treatment until they have determined the patient’s molecular profile.
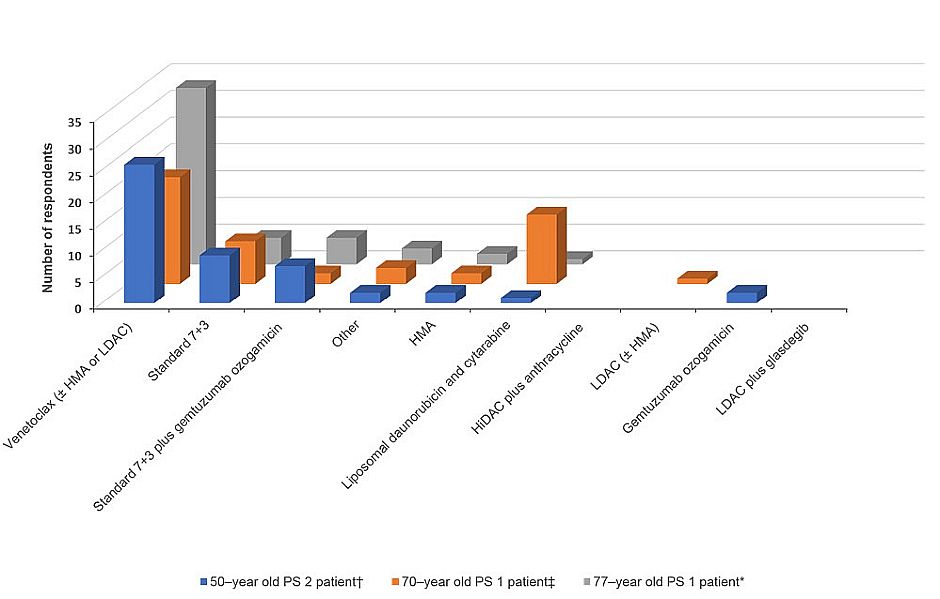
With the plethora of frontline treatment options now available, we were curious to gain insight into how patients with varying clinical profiles are being treated by community oncologists. A number of different patient scenarios were posed to provide a snapshot of the current practice patterns in managing AML. Venetoclax with or without hypomethylating agents (HMAs) or low-dose cytarabine (LDAC) was the most commonly recommended induction regimen for a number of different patient cases
- 77-year-old patient with performance status (PS) 1 and intermediate-risk AML (CD33 positive and without FLT3 mutation)
- 50-year-old patient with PS 2 who has a history of cardiovascular disease, including a previous heart attack, with intermediate-risk AML (CD33 positive and without FLT3 mutation)
- 70-year-old patient with PS 1 and therapy-related AML following treatment for mantle cell lymphoma (including autologous stem cell transplant [ASCT]), whose genomic profile was unknown
Other treatment options favored for these patients are featured in Figure 2. Interestingly, high-dose cytarabine (HiDAC) plus anthracycline and LDAC plus glasdegib seem to have fallen out of favor, with no physicians recommending these treatments for any of the 3 patients described above.
Figure 2. Summary of the frontline treatment recommendations for 3 different patient cases
*Seventy-seven-year-old PS 1 patient with intermediate-risk AML (CD33 positive and without FLT3 mutation).
†Fifty-year-old PS 2 patient who has a history of cardiovascular disease, including a previous heart attack, with intermediate-risk AML (CD33 positive and without FLT3 mutation).
‡Seventy-year-old PS 1 patient with therapy-related AML following treatment for mantle cell lymphoma (including autologous stem cell transplant); genomic profiling is unknown.
The picture was less clear for a 70-year-old PS 2 patient with intermediate-risk AML and IDH1 mutation. Ivosidenib plus HMA (33%), venetoclax with or without HMA or LDAC (27%), and ivosidenib (20%) were the 3 most favored treatment regimens, while 10% of oncologists would recommend LDAC plus glasdegib or ivosidenib plus 7+3. Finally, when considering AML patients who harbor FLT3 mutations, the majority of oncologists (59%) agreed they would use standard induction chemotherapy plus midostaurin, whenever feasible, regardless of other risk factors. These results should be interpreted with some caution, as 24% of oncologists have not treated a patient with a FLT3 mutation since midostaurin was approved.
In total, 98% anticipate that the use of venetoclax for newly diagnosed adult AML patients who are ≥75 years of age, or who have comorbidities that preclude use of intensive induction chemotherapy, will increase over the next 12 months. The accuracy of this prediction seems to be strengthened when considering the responses from oncologists regarding how many patients they have administered the following drugs to as a frontline treatment in the past 24 months: liposomal daunorubicin and cytarabine, gemtuzumab ozogamicin, midostaurin, gilteritinib, enasidenib, ivosidenib, glasdegib, and venetoclax. By far the most popular drug was venetoclax, which has been used in 163 patients during the last year, compared with 37 patients for the next most popular drug, midostaurin.
Practice patterns regarding venetoclax usage reveal that the majority of physicians use a daily dose ramp-up (71%), commonly coadministered with prophylactic antifungals (73%), and modify the venetoclax dose on the basis of prophylactic antifungal use (96%).
Management of R/R disease
Repeat mutational profiling is considered important in choosing the most appropriate treatment for patients with R/R AML.
Treatment recommendations for patients with R/R AML are more varied, although venetoclax remains a popular choice.
The final segment of the meetings concerned the management of patients with R/R AML. All but 1 participant would perform repeat biomarker testing for patients with R/R AML, with 88% opting for a full biomarker analysis, while 19% would only test for specific biomarkers. The oncologists were also asked to provide their treatment recommendations for different patient scenarios in the R/R setting. The first featured a 52-year-old female who has inversion 16 AML and had completed induction with standard 7+3, and 4 cycles of consolidation with HiDAC. After 18 months, the patient relapsed with AML and inversion 16; she was assumed to have PS 0 and no major comorbidities. In this setting, over 50% of oncologists stated they would refer the patient to an academic center for a bone marrow transplant evaluation and a decision regarding reinduction therapy. The second most common treatment choice was a repeat course of the 7+3 regimen or treatment with fludarabine-cytarabine-granulocyte colony-stimulating factor (FLAG)–idarubicin (14% each).
The second patient scenario was a 58-year-old male with FLT3-ITD–mutated AML (allelic ratio 0.55) who had received induction and 4 consolidations with 7+3 plus midostaurin. The patient had declined ASCT and did not receive midostaurin maintenance therapy. Five months after his last consolidation, the patient relapsed. Blood biochemistry results revealed his white blood cell count was 12,000, hemoglobin levels were 9.2 g/dL, and the platelet count was 42,000; the PS was 1 and the patient had no acute symptoms. There was no clear consensus among the oncologists on how they would treat this patient. Twenty-four percent of participants stated they would repeat the FLT3 mutation analysis, requesting a rush on the test. The next most popular management strategies were initiation of gilteritinib therapy, treatment with FLAG-idarubicin followed with allogeneic stem cell transplantation, or administration of venetoclax with HMA (22% each), while 8% opted to reinduce with 7+3 plus midostaurin.
The final question concerned which drugs had been used to treat patients with R/R AML during the past 24 months. Venetoclax was by far the most prolifically used treatment, with 97 patients having been treated during the last 2 years. The uptake of other drugs was much lower: 18 patients had been treated with midostaurin or gemtuzumab ozogamicin; 16 patients had received gilteritinib; 15 had received liposomal daunorubicin and cytarabine, or ivosidenib, while only 6 patients had received either enasidenib or glasdegib.
In summary, risk stratification, including mutational profiling, is critical to determining the optimal treatment strategies for patients with AML. Regimens featuring venetoclax are the most common frontline treatment for patients with AML and a popular choice for the appropriate patients in the R/R setting.